In the early hours of 29 December, NEJM published online a new clinical phase III study of the new Chinese coronavirus VV116. The results showed that VV116 was no worse than Paxlovid (nematovir/ritonavir) in terms of duration of clinical recovery and had fewer adverse events.
Image source:NEJM
Median recovery time 4 days, adverse event rate 67.4%
VV116 is an oral nucleoside anti-new coronavirus (SARS-CoV-2) drug developed in collaboration with Junsit and Wang Shan Wang Shui, and is an RdRp inhibitor together with Gilead’s remdesivir, Merck Sharp & Dohme’s molnupiravir and Real Biologics’ azelvudine.
In 2021, a phase II clinical trial of VV116 was completed in Uzbekistan. The results of the study showed that the VV116 group could better improve clinical symptoms and significantly reduce the risk of progression to the critical form and death compared to the control group. Based on the positive results of this trial, VV116 has been approved in Uzbekistan for the treatment of patients with moderate-to-severe COVID-19, and has become the first new oral coronary drug approved for marketing overseas in China [1].
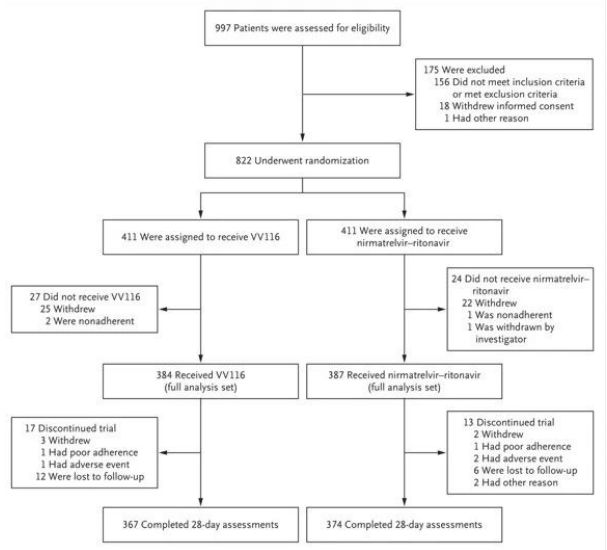
This phase III clinical trial[2] (NCT05341609), led by Prof. Zhao Ren of Shanghai Ruijin Hospital, Prof. Gaoyuan of Shanghai Renji Hospital and Academician Ning Guang of Shanghai Ruijin Hospital, was completed during the outbreak caused by the Omicron variant (B.1.1.529) from March to May in Shanghai, with the aim of evaluating the efficacy and safety of VV116 versus Paxlovid for the early treatment of patients with mild to moderate COVID-19. The aim was to evaluate the efficacy and safety of VV116 versus Paxlovid for the early treatment of patients with mild to moderate COVID-19.
Image source: Reference 2
A multicentre, observer-blinded, randomised, controlled trial of 822 adult Covid-19 patients at high risk of progression and with mild to moderate symptoms was conducted between 4 April and 2 May 2022 to assess the eligibility of participants from seven hospitals in Shanghai, China. Ultimately, 771 participants received either VV116 (384, 600 mg every 12 hours on day 1 and 300 mg every 12 hours on days 2-5) or Paxovid (387, 300 mg nimatuvir + 100 mg ritonavir every 12 hours for 5 days) as oral medication.
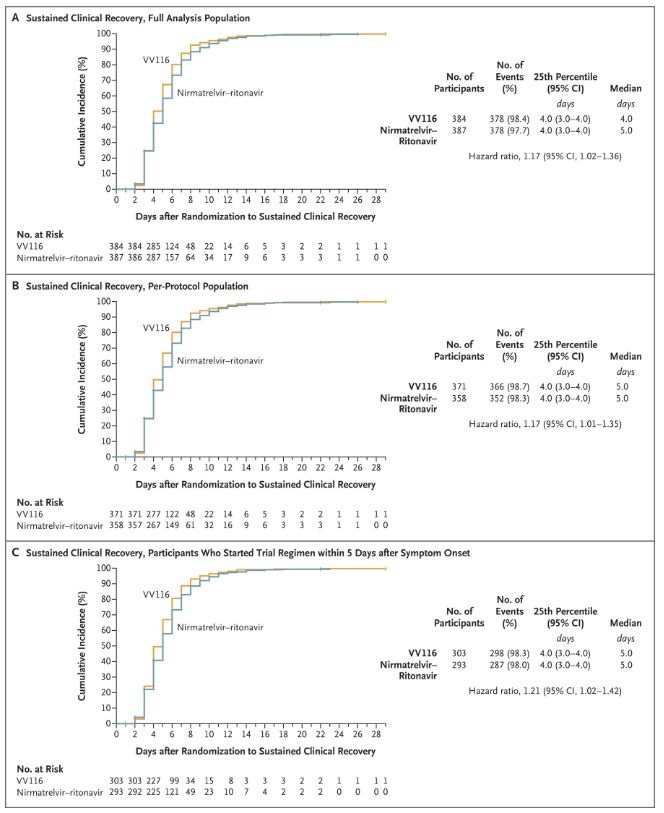
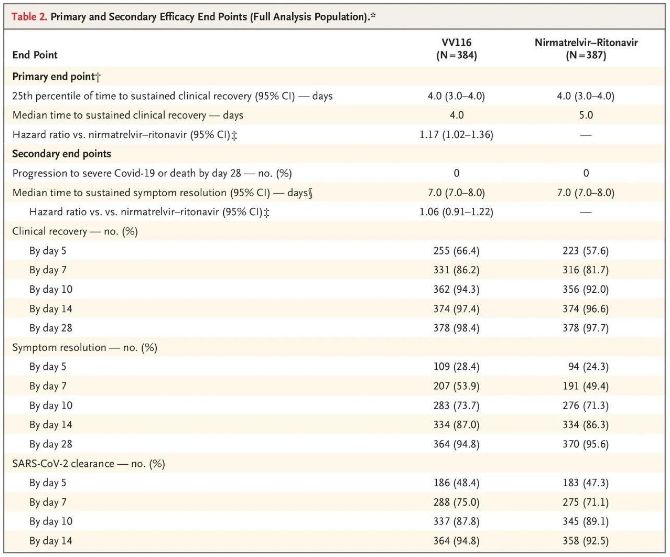
The results of this clinical study showed that early treatment with VV116 for mild to moderate COVID-19 met the primary endpoint (time to sustained clinical recovery) predicted by the clinical protocol: median time to clinical recovery was 4 days in the VV116 group and 5 days in the Paxlovid group (hazard ratio, 1.17; 95% CI, 1.02 to 1.36; lower limit. >0.8).
Maintaining clinical recovery time
Primary and secondary efficacy endpoints (comprehensive analysis of the population)
Image source: Reference 2
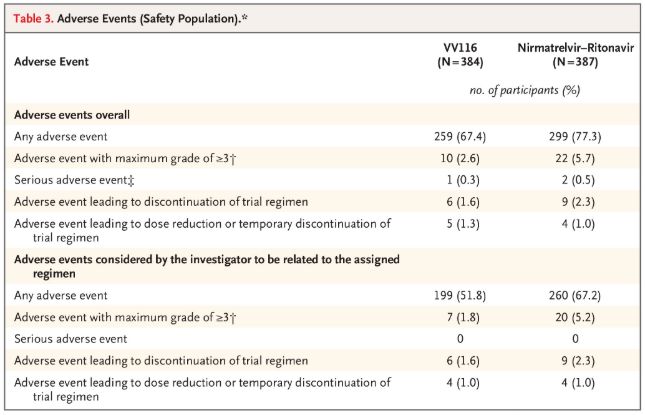
In terms of safety, participants receiving VV116 reported fewer adverse events (67.4%) than those receiving Paxlovid (77.3%) at the 28-day follow-up, and the incidence of Grade 3 / 4 adverse events was lower for VV116 (2.6%) than for Paxlovid (5.7%).
Adverse events (safe people)
Image source: Reference 2
Controversies and questions
On May 23, 2022, Juniper disclosed that the Phase III registration clinical study of VV116 versus PAXLOVID for the early treatment of mild to moderate COVID-19 (NCT05341609) met its primary study endpoint.
Image source: Reference 1
At a time when details of the trial were lacking, the controversy surrounding the Phase III study was twofold: firstly, it was a single-blind study and, in the absence of a placebo control, it was feared that it would be difficult to judge the drug completely objectively; secondly, there were questions about the clinical endpoints.
The clinical inclusion criteria for Juniper are (i) positive results for the new crown test, (ii) one or more mild or moderate COVID-19 symptoms, and (iii) patients at high risk of severe COVID-19, including death. However, the only primary clinical endpoint is ‘time to sustained clinical recovery’.
Just prior to the announcement, on May 14, Juniper had revised the clinical endpoints by removing one of the clinical primary endpoints, “proportion of conversions to serious illness or death” [3].
Image source: Reference 1
These two main points of contention were also specifically addressed in the published study.
Due to the sudden outbreak of Omicron, production of placebo tablets for Paxlovid had not been completed prior to the start of the trial and therefore the investigators were unable to conduct this trial using a double-blind, double-mock design. As for the single-blind aspect of the clinical trial, Juniper said that the protocol was conducted after communication with regulatory authorities and that the single-blind design means that neither the investigator (including the evaluator of the study endpoint) nor the sponsor will know the specific therapeutic drug allocation until the final database is locked at the end of the study.
Up to the time of the final analysis, none of the participants in the trial had experienced death or progression to a severe Covid-19 event, so no conclusions can be drawn about the efficacy of VV116 in preventing progression to severe or critical Covid-19 or death. The data indicated that the estimated median time from randomisation to sustained regression of Covid-19-related target symptoms was 7 days (95% CI, 7 to 8) in both groups (hazard ratio, 1.06; 95% CI, 0.91 to 1.22) [2]. It is not difficult to explain why the primary endpoint of ‘rate of conversion to severe illness or death’, which was originally set before the end of the trial, was removed.
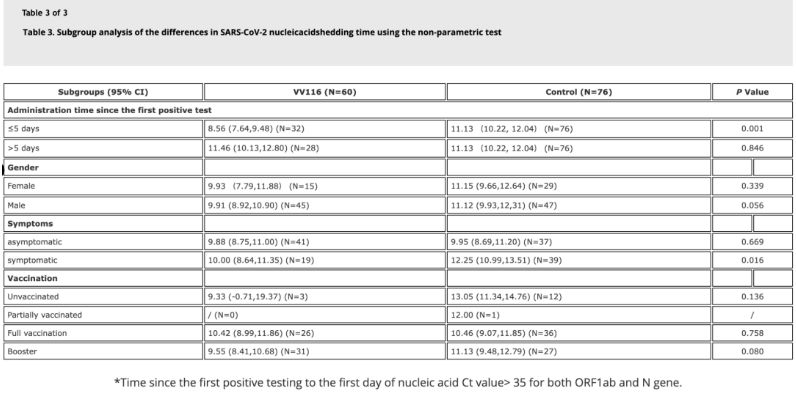
On 18 May 2022, the journal Emerging Microbes & Infections published the results of the first clinical trial of VV116 in patients infected with the Omicron variant [4], an open, prospective cohort study with 136 confirmed inpatients.
Data from the study showed that patients with Omicron infection who used VV116 within 5 days of their first positive nucleic acid test had a time to nucleic acid regression of 8.56 days, less than the 11.13 days in the control group. Administration of VV116 to symptomatic patients within the timeframe of this study (2-10 days of first positive nucleic acid test) reduced the time to nucleic acid regression in all patients. In terms of drug safety, no serious adverse effects were observed in the VV116 treatment group.
Image source: Reference 4
There are three ongoing clinical trials on VV116, two of which are phase III studies on mild to moderate COVID-19 (NCT05242042, NCT05582629). The other trial for moderate to severe COVID-19 is an international multicentre, randomised, double-blind phase III clinical study (NCT05279235) to evaluate the efficacy and safety of VV116 compared to standard treatment. According to the announcement by Juniper, the first patient was enrolled and dosed in March 2022.
Image source:clinicaltrials.gov
References:
[1]Junshi Biotech: Announcement on the main end point of Phase III registered clinical study of VV116 versus PAXLOVID for early treatment of mild to moderate COVID-19
[2]https://www.nejm.org/doi/full/10.1056/NEJMoa2208822?query=featured_home[3]https://clinicaltrials.gov/ct2/show/record/NCT05341609[4] Ensi Ma, Jingwen Ai, Yi Zhang, Jianming Zheng, Xiaogang Gao, Junming Xu, Hao Yin, Zhiren Fu, Hao Xing, Li Li, Liying Sun, Heyu Huang, Quanbao Zhang, Linlin Xu, Yanting Jin, Rui Chen, Guoyue Lv, Zhijun Zhu, Wenhong Zhang, Zhengxin Wang. (2022) Omicron infections profile and vaccination status among 1881 liver transplant recipients: a multi-centre retrospective cohort. Emerging Microbes & Infections 11:1, pages 2636-2644.
Post time: Jan-06-2023
 中文网站
中文网站